


|
ReGena-Vet Laboratories, LLC |
|
The Best Medicine for Our Best Friends |
|
Tomorrow’s Cures Today |

|
Ongoing Clinical Trials 6 |
|
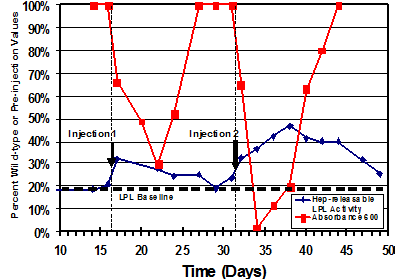
3. Other Genetic Deficiencies: Lipoprotein Lipase Deficiency (LPL-/-) Recruiting patients The first animal that we tested with allogeneic donor stem cells to test for their ability to correct a genetic defect was the lipoprotein lipase-deficient (LPL-/-) cat. In this model of genetic metabolic disease, serum is very opaque due to the animal’s inability to breakdown circulating chylomicrons (the role of the deficient LPL). In the photo in the upper right, pre- and post-injection serum from an injected cat are shown against a contrasting background. From the right, samples 1, 3 and 6 are pre-treatment and 2, 4 and 5 are post-treatment samples. They are placed approximately above where the serum opacity was measured. Serum opacity is quantified by light transmittance (A600) and presented in the diagram (right) (red solid line). After each injection, the absorption decreased significantly. |

|
Co-incidentally, measured LPL activity increased (solid blue line) documenting the ability of allogeneic cells to replace the defective gene product in this LPL-/- cat. This data documents that ability of allogeneic stem cells to correct heritable genetic defects. Based on the response of this cat to allogeneic MSCs, we have begun testing our allogeneic stem cells in a variety of feline and canine heritable diseases. |
